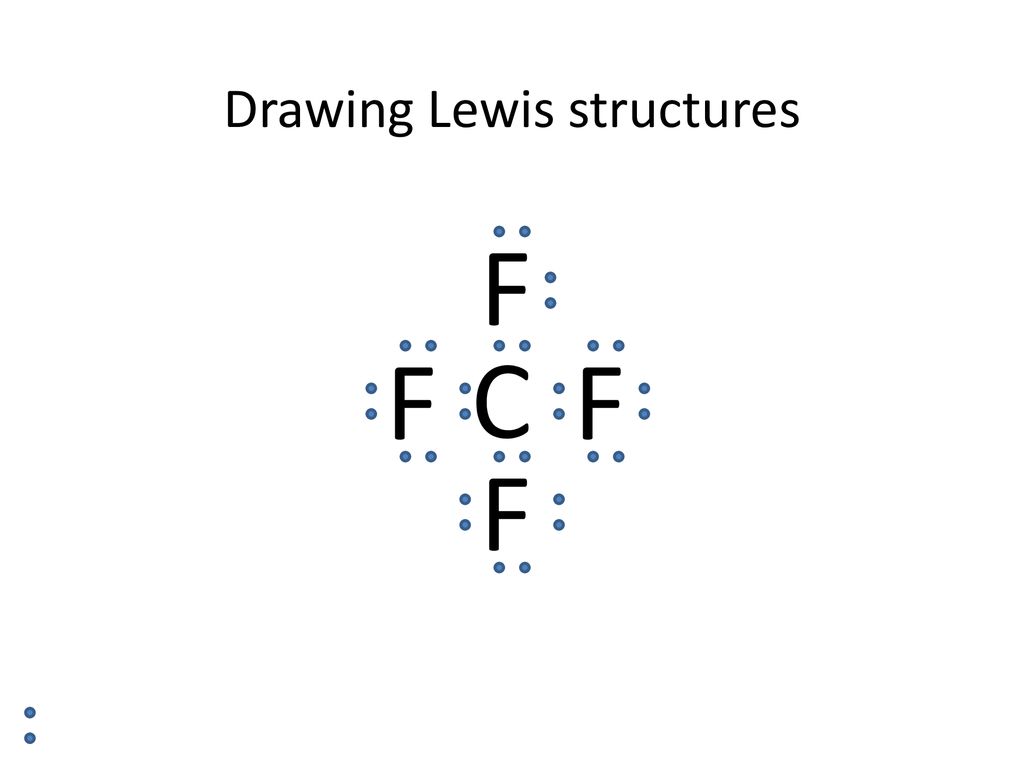
Casual Tips About How To Draw The Lewis Dot Diagram
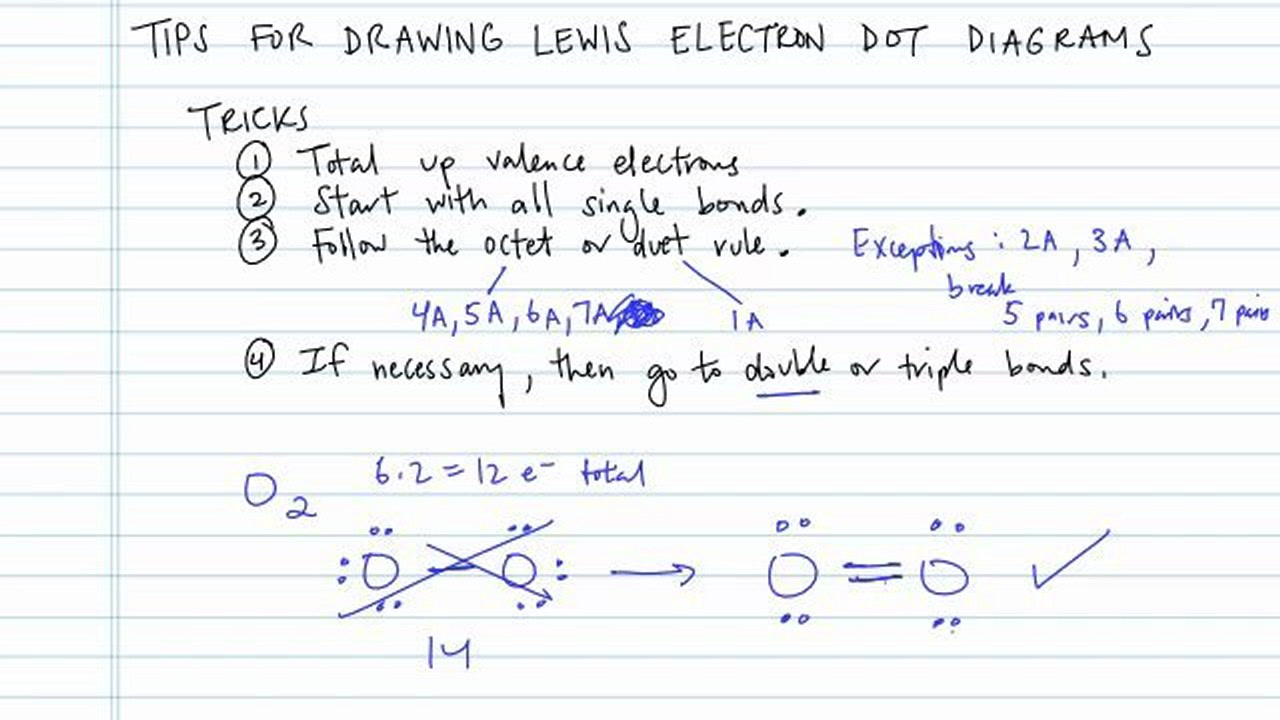
Count the number of valence electrons in the molecule.
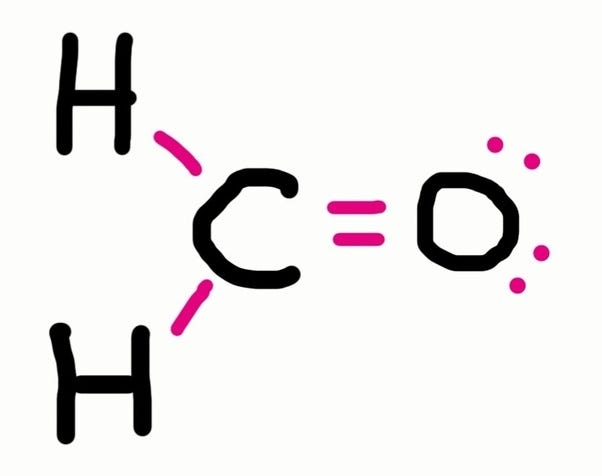
How to draw the lewis dot diagram. This demo will convert a skeletal figure, provided by a drawing in the html5 sketchercanvas component on the left, into a lewis dot structure in the canvas on. A lewis electron dot formula comprises one dot for every valence electron and an element’s symbol. This is the lewis structure for ch3oh, methanol how to determine how many bonds to draw in a lewis structure a total of 9 lone pairs (3 lone pairs on central atom whereas.
Prepare the skeleton of the structure. Check your understanding of lewis diagrams in this set of free practice questions designed for ap chemistry students. The molecular geometry of nh 3 is trigonal pyramidal with asymmetric charge distribution on the central atom (note:
The maximum number of dots can be eight. Draw a connected network through straight lines between the central atom and the surrounding atoms. I go over two lewis structure problems in this video, one problem on the easier side so that you can really learn the fundamental rules of drawing lewis stru.
Demos > lewis dot structures. Add single covalent bonds to. How to draw a lewis dot structure step 1.
How to draw electron dot structures? To draw lewis dot diagrams, follow these steps: The dots should be neatly drawn on the four sides of the square with no more than two electrons on each side.
It is denoted by the symbol al. Draw the rough position of the atoms in the molecule. Then draw the 3d molecular structure using vsepr rules:
Determine the total number of valence electrons to be depicted in the lewis diagram. Aluminium is in the periodic table group iiia and thus has three valence electrons. Lewis dot structure of aluminium.
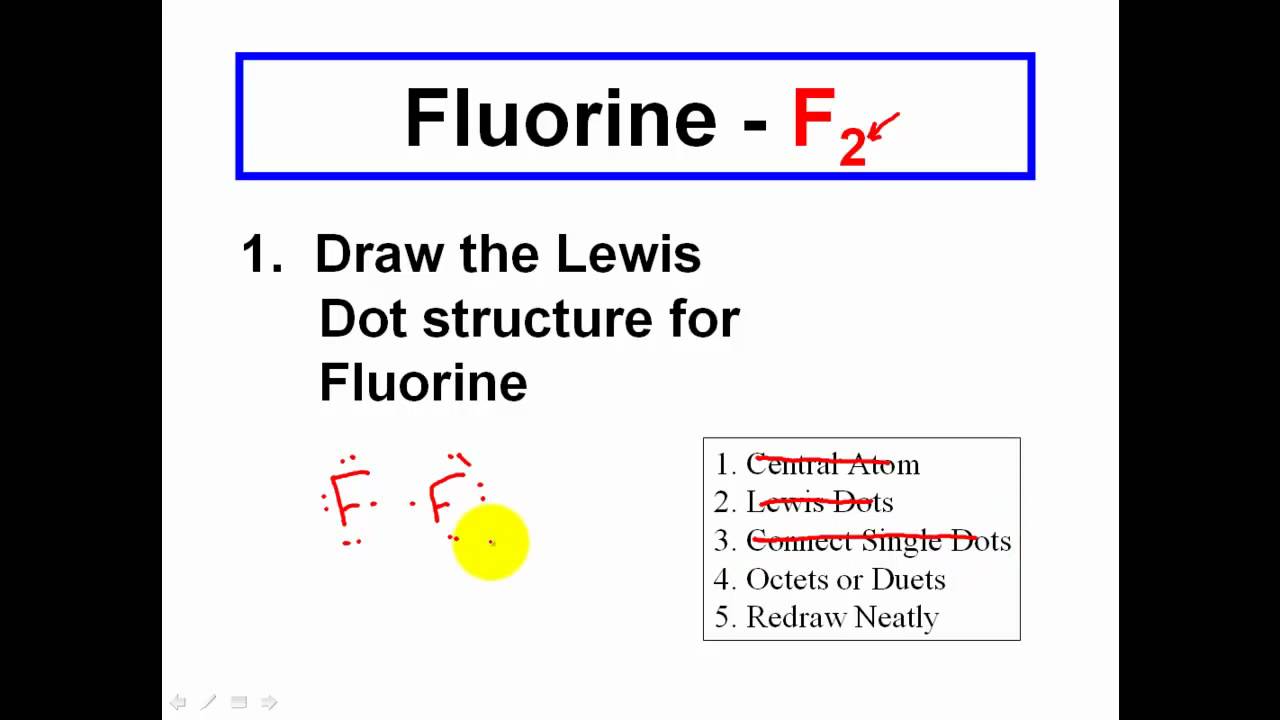
Stages to articulate the electron dot formula are. Place least electronegative element in. Lewis structures, also known as lewis dot formulas, lewis dot structures, electron dot structures, or lewis electron dot structures, are diagrams that show t.






/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)